Faculty A-Z

Graham M. Fraser
Associate Professor of Cardiovascular Sciences PhD (University of Western Ontario)BioMedical Sciences
709-864-4313
Email:
graham.fraser@mun.ca
Address:
Health Sciences Centre Room 5337 Division of BioMedical Sciences
Faculty of Medicine Memorial University of Newfoundland
300 Prince Philip Drive St. John's, NL, CANADA A1B 3V6
RESEARCH PROGRAM
Mass Transport and the Microcirculation: The nutritive requirements of all body tissues are ultimately provided for by the dynamic distribution of blood flow between microvascular networks. Under normal physiological conditions this distribution is modulated by metabolic demands of the surrounding tissue in response to changes both within microvessels and the surrounding local microenvironment. My lab aims to quantify mass transport of oxygen, nutrients, and other biologically active molecules from the vascular compartment into living tissue. Further, we are working to understand the integrated mechanisms behind blood flow regulation from the arteriolar level, through to capillary networks, and the constituent venous vasculature.
Microvascular Blood Flow: Fundamentally blood behaves as a non-Newtonian fluid with properties that change depending on hematocrit, shear, the composition of plasma, and the radius of blood vessels. The unique properties of blood make quantifying blood blow based on classic hydrodynamic laws difficult, particularly as the size of blood vessels transition within the microcirculation. On larger scales blood flow can be approximated as a homogenous fluid whereas in vessels smaller than 300 microns the two-phase nature of blood (cells and plasma) begins to have a much more significant impact on flow and flow distribution. In order to properly quantify blood flow in vivo it is necessary to make direct measurements from the vessels of interest.
Research Methods: Our primary methodology involves imaging microvascular networks using in vivo video microscopy. By using high-powered light microscopes we are able to measure vascular geometry, hemodynamics, and oxygen saturation within skeletal muscle microcirculation of living animals. Using this approach we can quantify mass transport of molecules of interest within the tissue. We use custom computer programs to process digital video and analyze the hemodynamics within capillaries. Videos can also be processed to reconstruct three-dimensional network geometries providing a detailed description of capillary and microvascular geometry. The vascular maps we generate are the most detailed representations of function capillary perfusion within skeletal muscle available. In vivo measurements from individual vessels are indexed to the corresponding vessels and used as inputs for mathematical models.
The oxygen transport models we use were developed by Dan Goldman at Western University and are based on well-defined physical laws of flow and diffusion. This ongoing collaboration allows for the calculation of convective and diffusive mass transport of a given molecule of interest between the vasculature and tissue. We have previously used these models to show how unsteady state in vivo data can be applied to steady state models of oxygen transport. These models have also been used to show how decreased functional capillary density in sepsis will impact oxygen delivery to tissue.
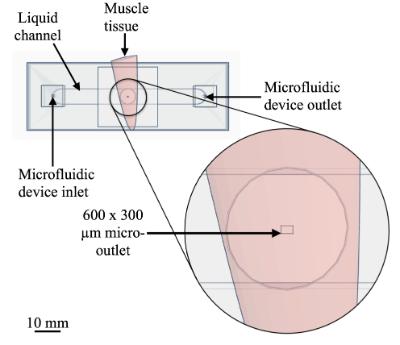
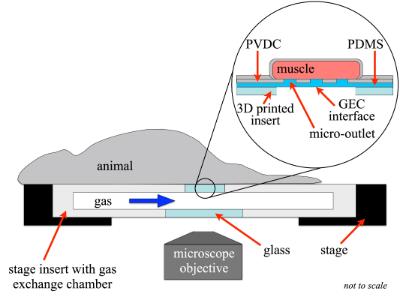
In order to further leverage the utility of intravital microscopy and computational modeling we are currently developing microfluidic devices to precisely manipulate tissue microenvironment in order to isolate specific mechanisms of microvascular regulation. This allows for the controlled introduction of drugs and probes to micron scale regions of tissue while directly observing the microcirculation using intravital video microscopy.
Disease Models: Currently our research is focusing on inflammatory disease and its impact on blood flow in capillary networks and the resulting defects to oxygen delivery, and glucose/insulin sensitivity in skeletal muscle. We use rodent models to quantitatively study vascular conditions in health, sepsis, type 2 diabetes, preeclampsia, and hypertension. My work on preeclampsia is an ongoing collaboration with the group at the University of Alberta (Craig Steinback, Margie Davenport, Jude Morton, and Sandra Davidge) where we are working to quantify the functional changes in capillary blood flow in the RUPP model of preeclampsia.
Other Research: I am also interested in cognitive spatial reasoning particularly in how it pertains to learning in anatomy and physiology. I have developed software to analyze spatial and temporal eye tracking data from spatial reasoning tasks in order to better understand how individuals process visual stimuli. This work is in collaboration with and Tim Wilson at Western University, and Victoria Roach at Oakland University.
FUNDING
Past Funding:
- NSERC Discovery Grant - Application of quantitative microfluidic devices to study mechanisms of microvascular blood flow regulation in vivo (2025-2030)
- CFI JELF - Advanced Imaging Facility for the Visualization of Live and Complex Biological Specimens (2022 – 2024)
- CIHR - Microvascular Blood Flow Regulation and Insulin Resistance in an Animal Model of Type 2 Diabetes (2019 - 2024)
- NSERC Discovery Grant - Quantitative Microfluidic Delivery Systems for Studying Tissue Microenvironment and Microvascular Blood Flow Regulation in Vivo (2017-2022)
- Medical Research Endowment Fund - Insulin Mediated Blood Flow Redistribution and Microvascular Insulin in an Animal Model of Type 2 Diabetes (2018-2021)
- Dean of Medicine Research Support Fund - Microvascular Blood Flow Regulation and Insulin Resistance in an Animal Model of Type 2 Diabetes (2018)
LAB MEMBERS
Graduate Students
- Alexander Bryce Folkins (M.Sc (Med)) – “Investigations into the O2-mediated mechanism of microvascular blood flow regulation in vivo: Carbon dioxide concentration dependence and role of adenosine triphosphate sensitive potassium ion channels” (2025)
- Gaylene Russell McEvoy (Ph.D.) – “Quantifying microvascular hemodynamics in healthy and type 2 diabetic rats” (2024)
- Meghan Kiley (M.Sc (Med)) – “The development and validation of ultra-thin-film micro-outlet devices for spatially constraining local O₂ perturbations to capillaries” (2023)
- Meaghan McCarthy (M.Sc (Med)) – “Characterization of CO₂-mediated microvascular blood flow responses in skeletal muscle tissue and investigation of underlying mechanisms using direct CO₂ microenvironment perturbations” (2022)
- Brenda Wells (M.Sc (Med)) – “Influence of local PO₂ on skeletal muscle microvascular blood flow during hyperinsulinemia” (2022)
- Gaylene Russell McEvoy (M.Sc (Med)) – “Development and validation of a novel microfluidic platform for studying local microvascular blood flow regulation in vivo” (2020)
Undergraduate Students
- Dalia Al-Badoosh (2025)
- Miya Burden (2025)
- Charlotte Campbell (2024-2025)
- Kanika Kaur (2022)
- Reily Smith (2020-2021)
- Hamza Shogan (2017-2021)
- Meghan Kiley (2019)
- Kaveh Mozafari (2018)
- Chelsey Broderick (2017)
RECENT PUBLICATIONS
Kiley ME, Sové RJ, Smith RH, Wells BN, Russell McEvoy GM, Fraser GM. Development of thin-film micro-outlets for spatially constraining local PO2 perturbations to capillaries in vivo. Front Physiol. 2025 Jul 9;16:1575776. doi: 10.3389/fphys.2025.1575776.
Russell McEvoy GM, Wells BN, Kiley ME, Shogan H, Fraser GM. Skeletal muscle microvascular hemodynamic responses during hyperinsulinemic-euglycemic clamp in a Zucker Diabetic Sprague Dawley rat model of type 2 diabetes. Front Physiol. 2025;16:1568145. Published 2025 Apr 23. doi:10.3389/fphys.2025.1568145
Wells BN, Russell McEvoy GM, Shogan H, Kiley ME, Fraser GM. Fixing skeletal muscle PO2 eliminates hyperinsulinemic microvascular blood flow response. Microcirculation. 2023;30(4):e12805. doi:10.1111/micc.12805
Wang A, Carlos A, Fraser GM, McGuire JJ. Endothelium dysfunction in hind limb arteries of male Zucker Diabetic-Sprague Dawley rats. Biochemical Pharmacology, 206, doi: 10.1016/j.bcp.2022.115319, 2022
Wang A, Carlos A, Fraser GM, McGuire JJ. The Zucker Diabetic Sprague Dawley (ZDSD) rat, an animal model for the study of type 2 diabetes. Experimental Physiology, 107(4): 265-282. doi: 10.1113/EP089947, 2022
Wang A, Fraser GM, McGuire JJ. Characterization of endothelium-dependent relaxation in the saphenous artery and its caudal branches in young and old adult Sprague Dawley rats. Biomolecules, 12(7), 889.
doi: 10.3390/biom12070889, 2022
Christie JR, Mawdsley LC, Kong I, Doornekamp AH, Baek JMC, Fraser GM, Ellis CG, Sové RJ. Novel Functional Imaging Technique to Determine Capillary Network Geometry and in vivo Capillary Hemoglobin Concentration in Skeletal Muscle. Microcirculation, Feb 11;e12751. doi: 10.1111/micc.12751, 2022
Russell McEvoy GM, Wells BN, Kiley ME, Kaur KK, Fraser GM. Dynamics of capillary blood flow responses to acute local changes in oxygen and carbon dioxide concentrations. Front Physiol. 2022;13:1052449. Published 2022 Dec 6. doi:10.3389/fphys.2022.1052449
Sové RJ, Milkovich S, Nikolov HN, Holdsworth DW, Ellis CG, Fraser GM. Localized Oxygen Exchange Platform for Intravital Video Microscopy Investigations of Microvascular Oxygen Regulation. Front Physiol. 2021;12:654928. Published 2021 Jun 8. doi:10.3389/fphys.2021.654928
Russell McEvoy GM, Shogan H, Sové RJ, Fraser GM. Development and validation of a novel microfluidic device for the manipulation of skeletal muscle microvascular blood flow in vivo. Microcirculation, 28(5):e12698.
doi: 10.1111/micc.12698, 2021
Ghonaim NW*, Fraser GM*, Goldman D, Yang J, Ellis CG. Regulation of oxygen supply at level of capillaries in skeletal muscle: response to localized oxygen exchange using a novel micro-delivery device. Microcirculation, 28(6):e12699. doi: 10.1111/micc.12699, 2021
Steele AR*, Skow RJ*, Fraser GM, Berthelsen LB, Steinback CD. Sympathetic neurovascular transduction following acute hypoxia. Clinical Autonomic Research, doi: 10.1007/s10286-021-00824-3, 2021
Tymko MM, Berthelsen LB, Skow RJ, Steele AR, Fraser GM, Steinback CD. Assessing dynamic and spontaneous neurovascular transduction using microneurography. Journal of Applied Physiology, 2021 doi: 10.1152/japplphysiol.00032, 2021
Steele AR, Berthelsen LB, Fraser GM, Phillips DB, Fuhr DP, Wong EYL, Stickland MK, Steinback CD. Blunted Sympathetic Neuro-cardiovascular Transduction is Associated to the Severity of Obstructive Sleep Apnea. Clinical Autonomic Research, 31(3):443-451. doi: 10.1007/s10286-021-00784-8, 2021
Skow RJ, Fraser GM, Steinback CD, Davenport MH. Prenatal Exercise and Cardiovascular Health (PEACH) Study: Impact on Muscle Sympathetic Nerve (Re)activity. Med Sci Sports Exerc, doi: 10.1249/MSS.0000000000002583, 2020
Skow RJ, Steele AR, Fraser GM, Davenport MH, Steinback CD. The sympathetic muscle metaboreflex is not different in the third trimester in normotensive pregnant women. The Journal of Applied Physiology, 130(3):640-650, doi: 10.1152/japplphysiol.00728, 2020
Berthelsen LB, Fraser GM, Simpson LL, Vanden Berg ER, Busch SA, Steele AR, Meah VL, Lawley JS, Figueroa-Mujica RJ, Vizcardo-Galindro GA, Villafuerte F, Gasho C, Wille CK, Tymko MM, Ainslie PN, Stembridge M, Moore JP, Steinback CD. Highs and Lows of Sympathetic Neurovascular Transduction: Influence of Altitude Acclimatization and Adaptation. American Journal of Physiology, 319:6, H1240-H1252, 2020 doi: 10.1152/ajpheart.00364, 2020
Coovadia Y, Adler TE, Steinback CD, Fraser GM, Usselman CW. Sex differences in dynamic blood pressure regulation: beat-by-beat responses to muscle sympathetic nerve activity. American Journal of Physiology,
doi: 10.1152/ajpheart.00245, 2020
Tymko MM, Fraser GM, Matenchuk BA, Day TA, Boulé NG, Davenport MH, Steinback CD. Determining whether sympathetic nervous activity influences cerebral blood flow at rest – A novel approach. Clin Auton Res, 30, 357–359 doi: 10.1007/s10286-020-00692-3, 2020
Akerstrom T, Goldman D, Nilsson F, Milkovich SL, Fraser GM, Brand CL, Hellsten Y, Ellis CG. Hyperinsulinaemia does not cause de novo capillary recruitment in rat skeletal muscle. Microcirculation, 27(2): e12593, 2020
McClatchey PM, Williams IM, Xu Z, Mignemi NA, Hughey CC, McGuinness OP, Beckman JA, Wasserman DH, Poole DC, Akerstrom T, Goldman D, Fraser GM, Ellis CG. Reply to Letter to the Editor: Perfusion controls muscle glucose uptake by altering the rate of glucose dispersion in vivo. Am J Physiol Endocrinol Metab, 318(3):E313-E317, 2019
Steinback CD*, Fraser GM*, Usselman CW, Skow RJ, Riske L, Julian CG, Stickland MK, Chari RS, Khurana R, Davidge ST, Davenport MH. Blunted Sympathetic Neurovascular Transduction during Normotensive Pregnancy. The Journal of Physiology, 597(14):3687-3696, 2019
Morton JS, Levasseur J, Quon A, Kirschenman R, Ganguly E, Dyck JD, Fraser GM*, Davidge ST*. Characterization of the Selective Reduced Uteroplacental Perfusion Model of Preeclampsia. Scientific Reports, 9(1), 9565. doi: 10.1038/s41598-019-45959-6, 2019
Sové, RJ, Drakos NE, Fraser GM, Goldman D, Ellis EG. Using digital inpainting to estimate incident light intensity for the calculation of red blood cell oxygen saturation from microscopy images. Journal of Biophotonics, p.201800103, 2018
Roach VA, Fraser GM, Kryklywy J, Mitchell D, Wilson TD. Guiding Low Spatial Ability Individuals Through Visual Salience Cueing: The Dual Importance of Where and When to Look. Anatomical Sciences Education, 10(6):528-537, 2018
Arpino JM, Nong Z, Li F, Yin H, Ghonaim N, Milkovich S, Balint B, O’Neil C, Fraser GM, Goldman D, Ellis CG, Pickering JG. 4D Microvascular Analysis Reveals That Regenerative Angiogenesis in Ischemic Muscle Produces a Flawed Microcirculation. Circulation Research, doi: 10.1161/CIRCRESAHA.116.310535, 2017
Roach VA, Fraser GM, Kryklywy JH, Mitchell DGV, Wilson TD. Time Limits in Testing: An analysis of eye movements, and visual attention in spatial problem solving. Anatomical Sciences Education, doi: 10.1002/ase.1695z, 2017
Sové RJ, Goldman, D, Fraser GM. A Computational Model of the Effect of Capillary Density Variability on Oxygen Transport, Glucose Uptake, and Insulin Sensitivity in Prediabetes. Microcirculation, 24 (2) p. e12342, 2017
Sové, RJ, Fraser GM, Goldman D, Ellis EG. Finite Element Model of Oxygen Transport for the Design of Geometrically Complex Microfluidic Devices Used in Biological Studies. PLoS One, 11(11), e0166289. doi: 10.1371/journal.pone.0166289.t001, 2016
Roach VA, Fraser GM, Kryklywy J, Mitchell D, Wilson TD. Different perspectives: spatial ability influences where individuals look on a timed spatial test. Anatomical Science Education, 10(3), 224–234. doi: 10.1002/ase.1654. 2016
Roach VA, Fraser GM, Kryklywy JH, Mitchell DGV, Wilson TD. The eye of the beholder: Can patterns in eye movement reveal aptitudes for spatial reasoning? Anatomical Sciences Education, 9(4), 357–366.
doi: 10.1002/ase.1583, 2016
Fraser GM, Morton JS, Schmidt SM, Bourque SL, Davidge ST, Davenport MH, Steinback CD. Reduced uterine perfusion pressure (RUPP) decreases functional capillary density in skeletal muscle. AJP: Heart and Circulatory Physiology, ajpheart.00641. 2015
Fraser, GM, Sharpe MD, Goldman D, Ellis CG. Impact of Incremental Perfusion Loss on Oxygen Transport in a Capillary Network Mathematical Model. Microcirculation. doi:10.1111/micc.12202, 2015