The APPROACH Study
APPROACH 2.0 Study participation and testing is now closed. You can find standard testing through a health care provider, a sexual health clinic, a local public health unit, or a walk-in clinic. Visit Action Canada for Sexual Health Resources: Find Sexual Health Services Near You
Background: What is APPROACH 2.0?
The Adaptation of Point-of-Care Testing (POCT) for Pharmacies to Reduce Risk and Optimize Access to Care in HIV, Hepatitis C, and Syphilis (APPROACH) study* was a three-year research project funded by the Canadian Institutes of Health Research (CIHR), focused on implementing sexually transmitted and blood-borne infections (STBBI) testing programs in community pharmacies.
Building on the success of the pilot APPROACH study (2017), APPROACH 2.0 was modified to offer testing for additional STBBIs, and aimed to improve equity and access to allow people options for where and how they receive testing services.
Community pharmacies offered a choice of point-of-care testing to screen for HIV, hepatitis C, and dried blood spot testing for HIV, hepatitis C, and syphilis as part of the research study. The goal was to implement a pharmacist testing program across three provinces to determine the effectiveness and acceptability to participants.
Between December 1, 2022, and March 31, 2024, The APPROACH Study recruited participants through select community pharmacies in three provinces: Newfoundland & Labrador (NL), Nova Scotia (NS), and Alberta (AB). In total, 77 pharmacists from 34 pharmacies—in both urban and rural locations—were part of the study.
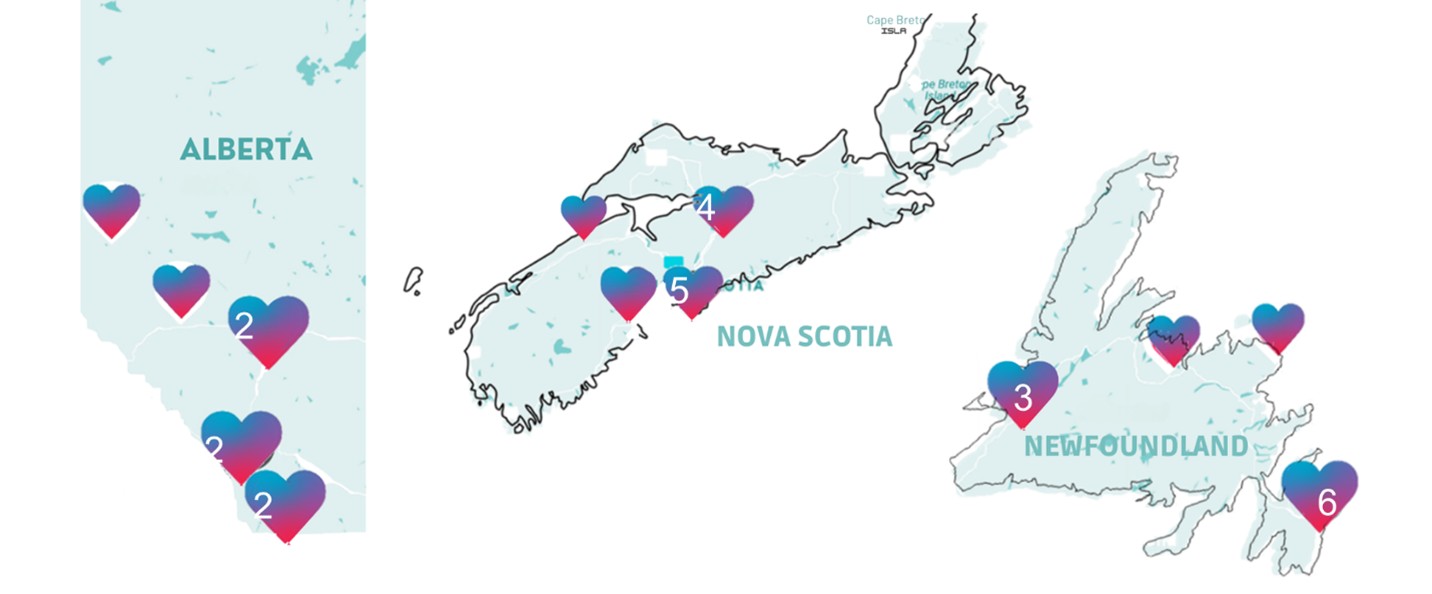

Those interested in taking part contacted participating pharmacies to arrange a testing visit. APPROACH participation with drop-in pharmacist testing was also offered at planned community events.
See what APPROACH Study parpticipation looked like.
All testing visit activities—including the consent process, counselling, and testing—were done in a private room. Participants were asked to answer anonymous surveys before and after the testing to collect information on demographics and their experiences with pharmacist-led testing. They were also invited to participate in telephone interviews held after the testing visit.
.jpg)
How It Worked
APPROACH testing: Participants were offered both point-of-care (POC) testing and/or dried-blood-spot (DBS) testing:
- A rapid point-of-care test (POCT) involved taking a few blood drops from a finger stick. The POCT for HIV can detect antibodies in 2 minutes. Results from the POCT to screen for Hepatitis C antibodies took 20 minutes. Results were shared and explained during the visit.
- A dried blood spot test (DBST) required a few blood drops from a finger stick, which were collected on a special paper, dried, and sent to a lab for testing. DBST screened for HIV, Hepatitis C, and/or syphilis from one sample. The pharmacist shared results when received through the lab, about 3-4 weeks after the visit.
Participants chose any combination of tests as part of the study. Pharmacists provided counselling during and after the testing visit as well as information on harm reduction and prevention, including HIV PrEP medication.
All participants with reactive results were given a blood work form to complete standard testing and referred to another health care provider (such as a physician or public health nurse) for follow-up care.
What We Learned
In all, 401 people participated in the APPROACH study, of which 399 were tested for at least one infection. A total of 1,424 tests were performed for the 399 participants.
Participants:
- ranged in age from 18 to 75 years old
- 57% identified as “Man” and 34% as “Woman”
- 34 % did not have or were unsure if they had access to a family doctor
For many participants, APPROACH testing was their first-time being tested for:
- HIV (35%),
- HCV (43%), and
- Syphilis (62%).
Over 46% of participants indicated that if pharmacist testing was not available that day, they would not go somewhere else (15%), or were unsure if they would go elsewhere (32%).
Approximately 11% reported that they had “nowhere else to get tested,” but this average is influenced by province (18% of responses from NS, 10% from NL, and 4% from AB).
What We Heard
Participants shared strongly positive responses about the pharmacist testing experience, indicating:
- they were comfortable (97%),
- were confident that the pharmacist was qualified (95%),
- did a good job administering the test (95%), and
- would recommend pharmacist testing to others (95%).
They shared that while there may have been some discomfort in the testing itself in terms of the fingerprick needed and anxiety over waiting for results, they were confident that the pharmacist was qualified and did a good job administering tests and sharing results. Participants described the pharmacist testing as convenient, easy to access, and a welcome option that should always be available.
Pharmacists also shared their perspectives on delivering the STBBI testing program in interviews partway through the study and focus groups after the study closed. They reported that APPROACH was received positively by patients and that their participation was a professionally rewarding experience. Pharmacists also shared challenges in recruiting people to the study, highlighting the need for ongoing promotion efforts. They also highlighted challenges and suggested ideas to inform future implementation of pharmacy-based STBBI testing.
In Summary
Pharmacist-led testing through the APPROACH 2.0 study was associated with:
- High participant satisfaction
- Success in reaching first-time testers, including those without a primary care provider
- Success in finding new infections and value of the testing interaction to provide education on how to reduce risk and prevent infections (data to come)
Participants felt strongly that pharmacist testing for HIV, HCV and syphilis should always be available, outside of the research study.
*The APPROACH 2.0 study is supported by the Canadian Institutes of Health Research (CIHR) through an HIV/AIDS Biomedical and Clinical Research Team Grant (HBR-422155).
With Research Ethics Board approval from: NL Health Research Ethics Board 2022.060 | Nova Scotia Health Research Ethics Board 1028410 | University of Alberta Health Research Ethics Board Pro00121218.
Research Team
Debbie Kelly, Principal Investigator & Research Team Lead, NL
Christine Hughes, Research Team Lead, AB
Amanda Butt, APPROACH Study Coordinator & Research Assistant, NL
Javiera Navarrete, Research Assistant, AB
Tasha Ramsey, Research Team Lead, NS
Mackenzie D’Entremont-Harris, Research Assistant, NS
For more information, please contact:
Dr. Debbie Kelly
709-864-7805
APPROACH Materials, References and Resources
Media
APPROACH Study Finding Success in De-Stigmatizing Sexual Health, says MUN Prof. VOCM News, February 28, 2024.
AIDS Committee Marks World AIDS Day. VOCM, December 1, 2023.
Island partners with researchers looking into STI testing at pharmacies. CBC News (PEI), November 20, 2023.
Let’s talk about sex(ual health). The Gazette, October 26, 2023.
Reducing Barriers to HIV Testing in HRM. Global News, February 7, 2023.
8 Alberta pharmacies to offer STI testing as part of U of A research study. CTV News, February 21, 2023.
New research study aims to make sexual health testing more accessible. University of Alberta Folio, February 21, 2013.
New research study aims to make sexual health testing more accessible in Alberta. University of Alberta News, February 21, 2023.
Grande Prairie included in APPROACH study. Everything GP, July 6, 2023.
Some Halifax pharmacies now offering HIV, hepatitis C, syphilis testing. CDKU Halifax’s Campus Radio Station, December 6, 2022.
New study will see Nova Scotia pharmacies test for HIV. CTV News, December 4, 2022.
Newfoundland pharmacies offering free STI testing amid doctor shortage. CBC News (NL), December 2, 2022.
Demand for STBBI Screening Significant in Rural NL. VOCM, December 2, 2022.
Know your status. Pharmacies 'ideally placed' to address gap in HIV, hepatitis C and syphilis testing. The Gazette, December 1, 2022.
New initiative making STI more accessible in Atlantic Canada. Global News (video), December 1, 2022.
New project brings HIV, Hep C and Syphilis testing to pharmacies in N.S. Global News, December 1, 2022.
New study launched on World AIDS Day will see free testing for HIV, hepatitis C and syphilis at local pharmacies. NTV News Feature (video). (Link no longer accessible)
NL Pharmacies to Offer Rapid Testing for Hepatitis C, HIV and Syphilis. VOCM Morning Show (Broadcast), December 6, 2021.
Conference Presentations
Kelly, D. et al. (2025) If we build it, will they come? Pharmacists’ perspectives on recruiting people to a pharmacy-based STBBI testing service (poster). European AIDS Clinical Society (EACS), Paris, France, 15-18 Oct 2025.
Navarrete, J. et al. (2025). “Pharmacists can do it": Experiences with offering testing for HIV, hepatitis C and syphilis through the APPROACH 2.0 study. Canadian Pharmacy Education and Research Conference (CPERC), Niagara Falls, Canada, June 17-20.
Kelly, D. (2025). Spotlight on the Atlantic Region: Challenges and Opportunities for HIV Research (Panel Delegate), Canadian Association for HIV Research Conference, Halifax, NS, May 4.
Ramsey, T. et al. (2025). Implementation of Pharmacist Testing For HIV, Hepatitis C, and Syphilis: The APPROACH 2.0 Study (oral). Canadian Association for HIV Research Conference, Halifax, NS, May 1-4.
Coombs et al. (2025). The Implementation of a Pharmacist-Led Point-of-Care Testing Service for HIV and Hepatitis C in Rural NL Correctional Facilities: A Pilot Study (oral). Canadian Association for HIV Research Conference, Halifax, NS, May 1-4.
Coombs et al. Acceptability of Pharmacist-Led Point-of-Care Testing for HIV and Hepatitis C in Correctional Facilities (poster). Canadian Association for HIV Research Conference, Halifax, NS, May 1-4.
Ramsey, T. (2025). Implementation of Pharmacist Testing for HIV, Hepatitis C, and Syphilis: The APPROACH 2.0 Study (presentation). Canadian HIV/AIDS Pharmacists Network (CHAP) AGM, Halifax, NS, May 1.
Hughes, C. et al. (2025). Testing, prevention, collaboration: A multi-methods study of a Canadian pharmacist-led model for HIV, HCV and syphilis testing (APPROACH 2.0). 83rd FIP World Congress of Pharmacy and Pharmaceutical Sciences, Copenhagen, Denmark, Aug 31-Sept 3.
Hughes, C. et al. (2024). Participants' experiences and acceptability of HIV, hepatitis C and syphilis testing by pharmacists in the APPROACH 2.0 study. Canadian Pharmacy Education and Research Conference (CPERC), Quebec City, Canada, June 11-14.
Kelly, D. et al. (2024). Pharmacy-based sexual health services are associated with high patient acceptability in Canada. Presented at the North American Conference on Integrated Care, Calgary, AB, Canada. International Journal of Integrated Care, 25(S2). https://ijic.org/articles/10.5334/ijic.NACIC24132
Kelly, D. (2024). STBBI testing by community pharmacists: Interim results from the APPROACH 2.0 study. Canadian Association for HIV Research Conference, London, Canada.
Kelly, D. & Norris, D. (2024). Acceptance of STBBI Testing by Pharmacists in the APPROACH 2.0 Study. Canadian Association for HIV Research Conference, London, Canada.
Kelly D. et al. (2023). Implementation of a pharmacy-based testing model in Canadian provinces: the APPROACH 2.0 study. Poster presented at the Canadian Association for HIV Research.
Warren, D., Kelly, D.V., Hatchette, T., Barrett, L. (2023). How has COVID-19 changed testing and linkages to care for communicable diseases (e.g., STBBIs, COVID-19)? What “lessons learned” would work well in the Atlantic region and why? What would be transferrable? How to scale up? Translating the lessons from the COVID-19 response to HIV & STBBI prevention - Atlantic Stakeholders Forum, Halifax, Canada.
Kelly, D. (2023). Pharmacy-based testing for STBBIs: Lessons in implementation from the APPROACH 2.0 study. Annual CHAP meeting, Quebec City, Canada.