Dr. Kurt Gamperl
Collaborators
Dr. Brian Dixon |
|
|
|
Canada Research Chair in Fish and Environmental ImmunologyDept. of Biology, University of WaterlooEmail: bdixon@uwaterloo.caWebsite: https://uwaterloo.ca/biology/people-profiles/brian-dixon |
Dr. Douglas A. Syme |
|
|
|
Department of Biological SciencesUniversity of Calgary, CanadaEmail: syme@ucalgary.caWebsite: http://bio.ucalgary.ca/bio_info/profiles/douglas-syme |
Dr. Felix Mark |
|
|
|
Division of Integrative EcophysiologyAlfred Wegener Institute, Bremerhaven , GermanyEmail: Felix.Christopher.Mark@awi.deWebsite: https://www.awi.de/ueber-uns/organisation/mitarbeiter/felix-christopher-mark.html |
Dr. Javier Santander |
|
|
|
Dept. of Ocean SciencesMemorial University of Newfoundland, CanadaEmail: jsanatendr@mun.caWebsite: http://www.faculty.mun.ca/jsantander/ |
Dr. Ken Rodnick |
|
|
|
Dept. of Biological SciencesIdaho State University, USAEmail: rodnkenn@isu.eduWebsite: http://www.isu.edu/bios/people/faculty/rodnkenn/ |
Dr. Matt Rise |
|
|
|
Ocean Sciences CentreMemorial University of Newfoundland, CanadaEmail: mrise@mun.caWebsite: https://www.mun.ca/osc/mrise/bio.php |
Dr. Stewart Johnson |
|
|
|
Department of Fisheries and Oceans,Pacific Biological StationE-mail: Stewart.Johnson@dfo-mpo.gc.caWebsite: https://profils-profiles.science.gc.ca/en/profile/stewart-johnson |
Contact Us
Memorial University of Newfoundland
St. Johns, NL
Canada, A1C5S7
Office: Room AX-4013
Phone: (709)864-2692
Fax: (709)864-3220
Email: kgamperl@mun.ca

Welcome to the Gamperl Laboratory
My lab maintains a broad interest in fish and environmental physiology, and marine finfish aquaculture, and takes an integrative multi-level approach to answer research questions in these areas. At present, there are 10 members of my lab, and these staff and students are an active, and integral, part of the research program.
Overview of the Research Program
The Ocean Sciences Centre (OSC) is ideally situated for studying the physiology and biology of coastal North Atlantic species, and provides easy access to fishes that live on the Grand Banks of Newfoundland. I utilize a number of OSC facilities in my research, in addition to my dedicated research labs/spaces, and these include:
1) The Dr. Joe Brown Aquatic Research Building which has state-of-the-art facilities for the rearing and culture of species such as cod, Atlantic salmon, lumpfish, steelhead trout, halibut etc.
 2) The Laboratory for Atlantic Salmon and Climate Change Research is a self-contained research facility with 10 (2.2 m3) and 8 (0.6 m3) tanks in which temperature, photoperiod and oxygen levels can be controlled / varied. This allows for short-term and long-term studies of fish physiology and immunology at temperatures from below 0oC to > 25oC.
2) The Laboratory for Atlantic Salmon and Climate Change Research is a self-contained research facility with 10 (2.2 m3) and 8 (0.6 m3) tanks in which temperature, photoperiod and oxygen levels can be controlled / varied. This allows for short-term and long-term studies of fish physiology and immunology at temperatures from below 0oC to > 25oC.
 3) The
Cold-Ocean and Deep-Sea Research Facility houses disease challenge labs and sophisticated scientific equipment (e.g. deep-sea chambers, Nikon confocal microscope with resonance scanning, Aria III flow cytometer / cell sorter, benchtop scanning EM, fully equipped histological suite etc.). This makes the OSC a great location to study marine fish physiology, immunology and disease processes, and the potential of various fish species for aquaculture production.
3) The
Cold-Ocean and Deep-Sea Research Facility houses disease challenge labs and sophisticated scientific equipment (e.g. deep-sea chambers, Nikon confocal microscope with resonance scanning, Aria III flow cytometer / cell sorter, benchtop scanning EM, fully equipped histological suite etc.). This makes the OSC a great location to study marine fish physiology, immunology and disease processes, and the potential of various fish species for aquaculture production.
At the OSC, my research program focuses on two main themes: 1) the environmental physiology of North Atlantic fish species; and 2) improving culture conditions for, and the health, welfare and disease resistance of, marine finfish cultured in Canada. This research uses a multi-level (telemetry, whole animal, organ/tissue, cellular, genomic etc.) approach to test hypotheses about how environmental conditions (e.g., temperature, oxygen, pressure, food availablity) affect metabolism, swimming performance, cardiovascular function and stress physiology, or how life history and ecology influence the design of physiological systems. In addition, I collaborate with Drs. Matt Rise and Javier Santander (OSC), and Dr. Brian Dixon (University of Waterloo), to investigate how various biotic and abiotic factors affect fish immunology.
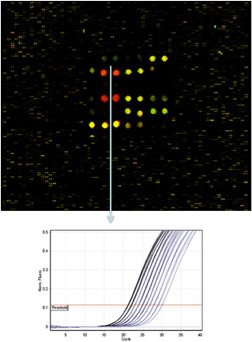
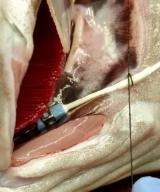
Techniques utilized in this research include: the implantation of physiological data loggers; respirometry for the measurement of fish metabolism/swimming ability; in vivo measurements of cardiovascular function, blood oxygen status and hormone levels; in situ measurements of heart performance; in vitro measurements of hormone receptors, enzyme activity and kinetics, and vascular function; OROBOROS flourorespirometry for the measurement of mitochondrial and cellular function; and qPCR and microarray analyses of gene expression.
 |
 |
 |
 |
 |
 |
||
Examples of Ongoing & Recent Research
Environmental Impact on Cardiovascular Function
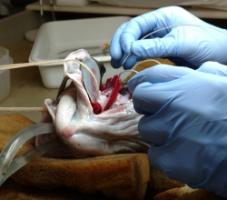
Over the past 40 years, substantial progress has been made in understanding the cardiovascular system of fish and how they control heart function and blood flow. However, we are far from completely understanding how their cardiovascular system deals with biotic or abiotic challenges, especially in combination. For example: 1) with the exception of a few Arctic and Antarctic species, we have a poor understanding of how the cardiovascular system of fishes functions at, or adapts to, cold (< 2ºC) temperatures; and 2) our understanding of what factors limit cardiac function at high temperatures has undergone considerable revision in the past few years, and it has become clear that there are inter-specific differences in fish cardiovascular function and how it responds to temperature change. Further, the cardiovascular response to temperature varies depending on water oxygen levels and depth (hydrostatic pressure). In this research, we are using a multi-level, integrated, approach to investigate how chronic and acute changes in temperature, oxygen levels and hydrostatic pressure (up to 100 bar; 1000 meters) affect the control and functional capacity of the cardiovascular system of North Atlantic and other fish species.

Aspects currently under investigation include:
1. How chronic high temperature and/or low oxygen affect the cardiorespiratory function of fishes; including mitochondrial function and its sensitivity to nitric oxide.
2. Swimming performance and in vivo and in vitro cardiovascular performance at cold temperatures.
3. How exposure to hydrostatic pressure affects fish heart rate responses to temperature and hypoxia.
Physiology of Cunner (Tautogolabrus adspersus)

Metabolic depression, or at least entrance into a dormant state characterized by the cessation of feeding and activity, has been observed in several marine and freshwater fish species when exposed to cold temperatures. The cunner is a western North Atlantic wrasse species that is at the northern limit of its distribution in Newfoundland, and has been observed to enter a period of physical inactivity, concomitant with decreased oxygen consumption rates and behaviour characteristic of a dormant state, in response to low water temperatures. However, it was unknown whether this was truly metabolic depression, and if so, what mechanisms might be involved. Thus, we initiated a research program to study various aspects of this species’ physiology. This research has:
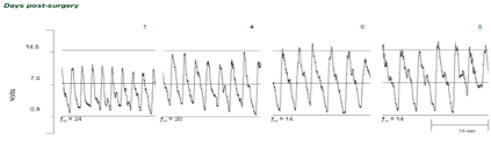
 1. Shown that the cunner goes into torpor (metabolic depression) when temperatures fall below 50C in the fall (i.e. seasonally), and investigated the contribution of diminished cardiac function to this metabolic depression;
1. Shown that the cunner goes into torpor (metabolic depression) when temperatures fall below 50C in the fall (i.e. seasonally), and investigated the contribution of diminished cardiac function to this metabolic depression;
2. Determined the capacity of cunner to deal with other environmental stressors (e.g. hypoxia; high temperatures);
3. Examined the contribution of changes in various cellular processes to metabolic depression. In addition, studies currently underway are focusing on how this species’ stress physiology differs from that of other marine teleosts.
Sablefish (Anaplopoma fimbria) Physiology and Immunology
The sablefish (Anoplopoma fimbria) is an interesting model for studying the environmental tolerances of marine fishes and the underlying determinants. It is widely distributed along the North Pacific, and while juveniles live in-shore, the adults inhabit cold and deep waters (up to 1,500 m) that are often severely hypoxic. For example, adult sablefish are known to colonize the oxygen minimum zone off the California coast where oxygen levels range from 0.34 to 0.80 mg O2 L-1. Furthermore, this species supports significant capture fisheries and is an emerging aquaculture species. With regards to the latter, Golden Eagle Sable Fish is currently rearing sablefish in British Columbia, and aims to reach an annual production of 500,000 juveniles and 1000+ tonnes of production in the next few years. As with any new (alternative) aquaculture species, husbandry and management procedures developed for other fish species will have to be modified for sablefish culture, and information on this species’ physiology, stress and disease resistance, and environmental tolerances must be gathered.
 Research conducted to date has determined the acute upper temperature and hypoxia tolerance of both adults and juveniles, and provided comprehensive information on this species’ stress and metabolic physiology. We are now developing genomic (i.e., molecular probes) and antibody-based tools (i.e., ELISAs) so that the immune function of this species can be studied, and work on the efficacy of vaccines against furunculosis (caused by the pathogen Aeromonas salmonicida) is ongoing.
Research conducted to date has determined the acute upper temperature and hypoxia tolerance of both adults and juveniles, and provided comprehensive information on this species’ stress and metabolic physiology. We are now developing genomic (i.e., molecular probes) and antibody-based tools (i.e., ELISAs) so that the immune function of this species can be studied, and work on the efficacy of vaccines against furunculosis (caused by the pathogen Aeromonas salmonicida) is ongoing.
Collaborators: Dr. Javier Santander (MUN), Golden Eagle Sable Fish (Briony Campbell, Tom Sorby, Megan Sorby), and Zoetis (Michael Ness and Nils Steine)
Mitigating the Impact of Climate-Related Challenges on Salmon Aquaculture (MICCSA)
In this pan-Atlantic applied research project, research tools and products are being developed to: enable the Atlantic salmon aquaculture industry to prepare for, and monitor, the predicted effects of warming and hypoxic coastal waters; and to develop fish that are better protected from infectious salmon anemia (ISA) and sea lice (Lepeophtheirus salmonis). These goals will be achieved by:
1. Defining the sub-lethal and lethal temperatures of current Atlantic salmon stocks (i.e., fish of Saint John River origin);
2. Studying how fish with varying genetic background perform under conditions of hypoxia and elevated temperature;
3. Developing molecular markers (single nucleotide polymorphisms, SNPs) for selecting broodstock with high temperature tolerance, robust immune responses and improved disease and stress resistance; and
4. Developing genomic and antibody-based diagnostic assays for assessing salmon health and producing improved, more effective, vaccines.
This research program has already: validated the use of data loggers that simultaneously record heart rate, activity/swimming speed and body temperature that can be used to monitor free-swimming Atlantic salmon in sea cages; provided significant data on how high temperatures (20-23oC) alone, or in combination with moderate hypoxia, impact production characteristics, stress physiology, and the salmon’s immune response to viral and bacterial antigens (i.e., vaccination); identified several key immune- and stress-related genes (biomarkers) that are responsive to temperature / hypoxic challenges; and produced antibodies and ELISAs (Enzyme-Linked Immunosorbent Assays) to several biomarkers so that their protein levels can be quantified and monitored.
Collaborators: Dr. Mark Fast (UPEI), Dr. Brian Dixon (University of Waterloo), Dr. Matt Rise (Memorial University), Dr. Roy Danzmann (University of Guelph), Danny Boyce (JBARB Facilities Manager), Dr. Amber Garber and Chris Bridger (Huntsman Marine Science Centre), Debbie Plouffe (Center for Aquaculture Technologies Canada), Jessi Rix (Somru BioScience)
Post-Doctoral Fellows |
||
Fabio Zanuzzo
|
Post-Doctoral Fellow (2015), São Paulo State University (UNESP) Ph.D. Aquatic Biology (2014), São Paulo State University (UNESP) M.Sc. Aquaculture (2010), São Paulo State University (UNESP) B.Sc. Biology, University (2008), São Paulo State University (UNESP) Email: fabioz@mun.ca |
Research: Fish Environmental Physiology, Immunology |
Julie Nati
|
Marie Curie Fellow (2021), UMR Marbec, CNRS, Ifremer, Montpellier, France Research Fellow (2018), University of Glasgow, UK Ph.D. Environmental and Evolutionary Biology (2016), University of Glasgow, UK M.Sc. Marine Environmental Protection (2010), Bangor University, Wales, UK M.Sc. General Oceanography, specialisation in Marine Biology and Ecology (2009), Aix-Marseille Université, Marseille, France B.Sc. Earth Sciences, specialisation in Marine Biology and Ecology (2008), Aix-Marseille Université, Marseille, France Email: jjhnati@mun.ca |
|
Ph.D. Students |
||
Robine Leeuwis |
M.Sc. Biology (2014), Radboud University Nijmegen, the Netherlands B.Sc. Biology (2012), Radboud University Nijmegen, the Netherlands Email: rhjleeuwis@mun.ca |
Research: Cardiovascular Physiology, Immunology and Nutrition of the Sablefish (Anoplopoma fimbria) |
Eric Ignatz
|
M.Sc. Aquaculture (2019), Memorial University Ontario College Graduate Certificate - Aquaculture (2016), Fleming College B.Sc.H. Animal Biology (2015), University of Guelph Email: ehignatz@mun.ca |
Research: Effects of Environmental and Nutritional Manipulations on Atlantic Salmon Physiology and Transcriptomics |
M.Sc. Students |
||
Émile Vadboncoeur
|
B.Sc. Biology (2017), Université du Québec à Rimouski Email: evadboncoeur@mun.ca |
Research: Effects of Cold Temperatures on Atlantic Salmon Physiology |
Emma Porter
|
B.Sc. Marine Biology (2019), Dalhousie University Email: esporter@mun.ca |
Research: Effects of Temperature Extremes on the Cardiorespiratory Physiology of Salmon |
Rachel Eisenberg
|
B.Sc. Zoology (2019), University of British Columbia (Okanagan) Email: reisenberg@mun.ca |
Research: Influence of Development temperature on the Physiology of Lumpfish |
Rebeccah Sandrelli |
B.Sc. Biology (Marine Biology) (2017), Memorial University Email: r.sandrelli@mun.ca |
Research:Biologgers as a Tool to Investigate the Thermal Biology of Fishes |
Darby Gielewski
|
B.Sc. Marine Biology (2021), Dalhousie University
|
|
Research Staff |
||
Kathy Clow |
B.Sc. Biology (1990), Dalhousie University Email: kclow@mun.ca |
Senior Research Assistant, Ocean Frontier Institute |
Rebeccah Sandrelli
|
B.Sc. Biology (Marine Biology) (2017), Memorial University Email: rmh486@mun.ca |
Research Assistant, MICCSA |
Tasha Harrold |
B.Sc. Agriculture, Environmental Biology, (2001), Dalhousie University/Nova Scotia Agricultural College Email: tlkharrold@mun.ca |
Project Manager for MICCSA and Module J of the Ocean Frontier Institute |
Recent Lab Members |
||
Anne Beemelmanns |
Post-Doc 2017 - 2020 Ph.D. Evolutionary Ecology of Marine Fishes (2016), Helmholtz Centre for Ocean Research, Kiel M.Sc. Aquatic Ecophysiology (2011), Heinrich-Heine-Universität Düsseldorf B.Sc. Biology (2009), Heinrich-Heine-Universität Düsseldorf Email: abeemelmanns@mun.ca |
Presently a Post-Doc with the Genome Canada funded FISH-GEN |
Lucie Gerber |
Post-Doc 2018-2020 Post-Doctoral Fellow (2017), University of Aarhus, Denmark Ph.D. Fish Physiology (2016), University of Southern Denmark, Denmark M.Sc. Biology (2013), University of Montpellier, France B.Sc. Biology (2011), University of Antilles, Guadeloupe Email: lgerber@mun.ca |
Presently a Post-Doc with Goran Nilsson, Oslo Norway |
Zoe Zrini
|
M.Sc. 2020 B.Sc. Zoology (2017), University of Guelph Email: zazrini@mun.ca |
Presently working with the Fish Culture Section, Ontario Ministry of Natural Resources |
Ellen Peroni |
Research Assistant 2017-2020 MBA in Agribusiness (2020), University of São Paulo (USP), Brazil M.Sc. Veterinary Medicine (2013), São Paulo State University (UNESP), Brazil B.Sc. Veterinary Medicine (2009), Franca University, Brazil Email: edfperoni@mun.ca |
|
Ashley Loveless
|
Aquaculture Technician, MICCSA 2019-2021 B.Sc. Biology (2020), Memorial University
|
|























