Research Highlights
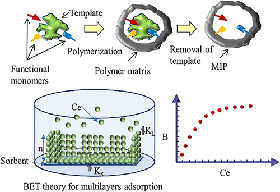
The detection of pollutants and contaminants in water is an essential part of protecting the natural environment and providing clean drinking water. Christina Bottaro's group develops Molecularly Imprinted Polymers that selectively absorb these compounds from water, allowing them to be detected with greater ease, sensitivity, and selectivity. A breakthrough development by their group was recently published in the leading materials chemistry journal, ACS Applied Materials and Interfaces (DOI: 10.1021/acsami.9b21493).
Graham Bodwell’s group is working the synthesis of severely distorted polycyclic aromatic hydrocarbons (PAH) and studying the effects of such distortion on the chemical and physical properties of the aromatic system. The largest aromatic system under investigation is teropyrene, which is a C36 decacyclic PAH.
Recent work has led to the development of a gram-scale synthesis of a [9](2,11)teropyrenophane, in which the teropyrene system is bent into almost a semicircle. Having access to synthetically useful amounts of material has enabled the exploration of the chemistry of the teropyrene system for the first time (Angew. Chem. Int. Ed. 2018, 57, 1707–1711). It was discovered that electrophilic aromatic substitution with molecular bromine readily affords a single tetrabromide. The very high regioselectivity of this reaction could be explained using the results of an EPR study of the easily-formed radical cation of the unbrominated cyclophane.
Several students in the Fran Kerton group have been developing polymerization catalysts based on earth abundant metals. In 2015, Hart Plommer (PhD candidate) saw his work on Aluminum catalysts published in Dalton Transactions. His catalyst showed an interesting structure-activity relationships for the ring-opening polymerization of epoxides to yield polyethers. With his most active catalyst, he was able to use an extremely low catalyst loading. He also designed the cover page for this special issue on earth-abundant elements in catalysis. The image in the background is a photo he took in Gros Morne National Park. Also in 2015, Dalal Alhashmialameer (PhD candidate) saw her work on ring-opening polymerization of lactide published in Dalton Transactions. Her report includes details on one of the most active sodium catalysts for this reaction to date. The product of her reactions poly(lactic acid) or PLA is a biodegradable polymer. Both of these papers are available free via an open-access license. Other researchers in Dr. Kerton's group work on carbon dioxide activation, biomass utilization and oxidation catalysis. Find our more about Green Chemistry @MUN by visiting our group's website.
The Katz group studies porous materials; an example of a porous material that we use every day is a sponge in our kitchen. The focus on the research program is to study the synthesis, properties, and applications of these materials. The main class of porous materials that the research team investigates are metal-organic frameworks (MOFs). MOFs are made from inorganic metal cations or clusters which are connected to one another via organic bridging ligands (e.g., terephthalic acid). The structures of MOFs are such that a large percentage of framework-free space (e.g., like the pockets in a sponge) is available for chemistry (e.g., like absorbing water in a sponge). Unlike the sponge example, one of the research themes within the group focuses on studying how environmentally harmful chemicals are absorbed into a porous material. With this in mind, the research team is a multidisciplinary team that combines organic, inorganic, and physical chemistry methods to address many challenges. In a related manuscript published in Chemical Communications (DOI: 10.1039/C5CC09919F), the research team studied how a particular class of MOFs changes as a function of time. By understanding how and why these materials change over time, we can understand how they will function in various applications. Recently, Dr. Katz has received the Terra Nova Young Innovator Award. This funding opportunity will allow the research team to begin to investigate a new class of porous materials.
The research group of Travis Fridgen is exploring the interactions of metal ions with biomolecules and the fundamental forces which give biomolecules their three-dimensional shape. Graduate and undergraduate research student's in Dr. Fridgen's group are using state-of-the-art instrumentation, combining ion-trapping mass spectrometry with tunable infrared lasers to spectroscopically probe the structures of these ions in the gas phase. Recently (DOI: 10.1021/jp809993k) they have shown that solvating metal-ion bound DNA bases results in a huge structural change, promoting intramolecular hydrogen bonding.