Peptide bond formation by an in vivo condensation reaction: a detailed account
In vivo formation of the peptide bond is
often described as a dehydration
reaction by analogy with the in vitro
reaction that splits out of an H20.
However, the in vivo
reaction is properly described as a
condensation reaction.
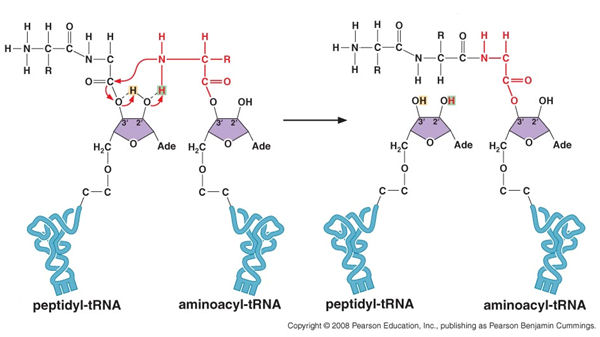
Recall that the C terminus of the amino acid in the P site is joined to the 3'-C of the terminal nucleotide at the end of the tRNA by an ( -O- ) ester bond. This means the C-terminus contributes to to the peptide bond ass a carbonyl radical (-C=O), without a separate -OH group. The peptide bond is formed by a nucleophilic attack of the N terminus of the amino terminus in the A site on this carbonyl C, while the ester bond to the tRNA remains intact. The proton on the 2'-C then shifts to the 3'-C so as to restore the -OH terminus of the now uncharged tRNA, and a proton from the N terminus of the A site amino acid the shifts to the 2'-C. The in vivo reaction balances, without production of an H20 molecule.
Recall that the C terminus of the amino acid in the P site is joined to the 3'-C of the terminal nucleotide at the end of the tRNA by an ( -O- ) ester bond. This means the C-terminus contributes to to the peptide bond ass a carbonyl radical (-C=O), without a separate -OH group. The peptide bond is formed by a nucleophilic attack of the N terminus of the amino terminus in the A site on this carbonyl C, while the ester bond to the tRNA remains intact. The proton on the 2'-C then shifts to the 3'-C so as to restore the -OH terminus of the now uncharged tRNA, and a proton from the N terminus of the A site amino acid the shifts to the 2'-C. The in vivo reaction balances, without production of an H20 molecule.
Figure after ©2008 by Watson et al.; All text material ©2014 by Steven M. Carr