Evolution of Myoglobin in diving mammals
Myoglobin and Hemoglobin are both O2
retaining and transporting molecules, with a common
evolutionary origin. Myoglobin is primarily a
muscle-storage molecule, whereas hemoglobin is
stored in red blood cells (rbcs) of the
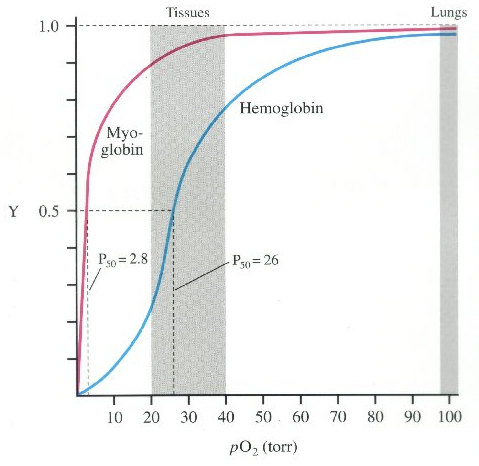
bloodstream. [Left] Hemoglobin releases O2 in
tissues at a much lower partial pressure (20 ~ 40 pO2)
than does Myoglobin. Diving mammals release O2
from Myoglobin only under extreme pressure during deep dives
(2.8 pO2).
Deep-diving animals also collapse their lungs, and restrict
blood circulation to the heart and CNS as behavioral
adaptation.
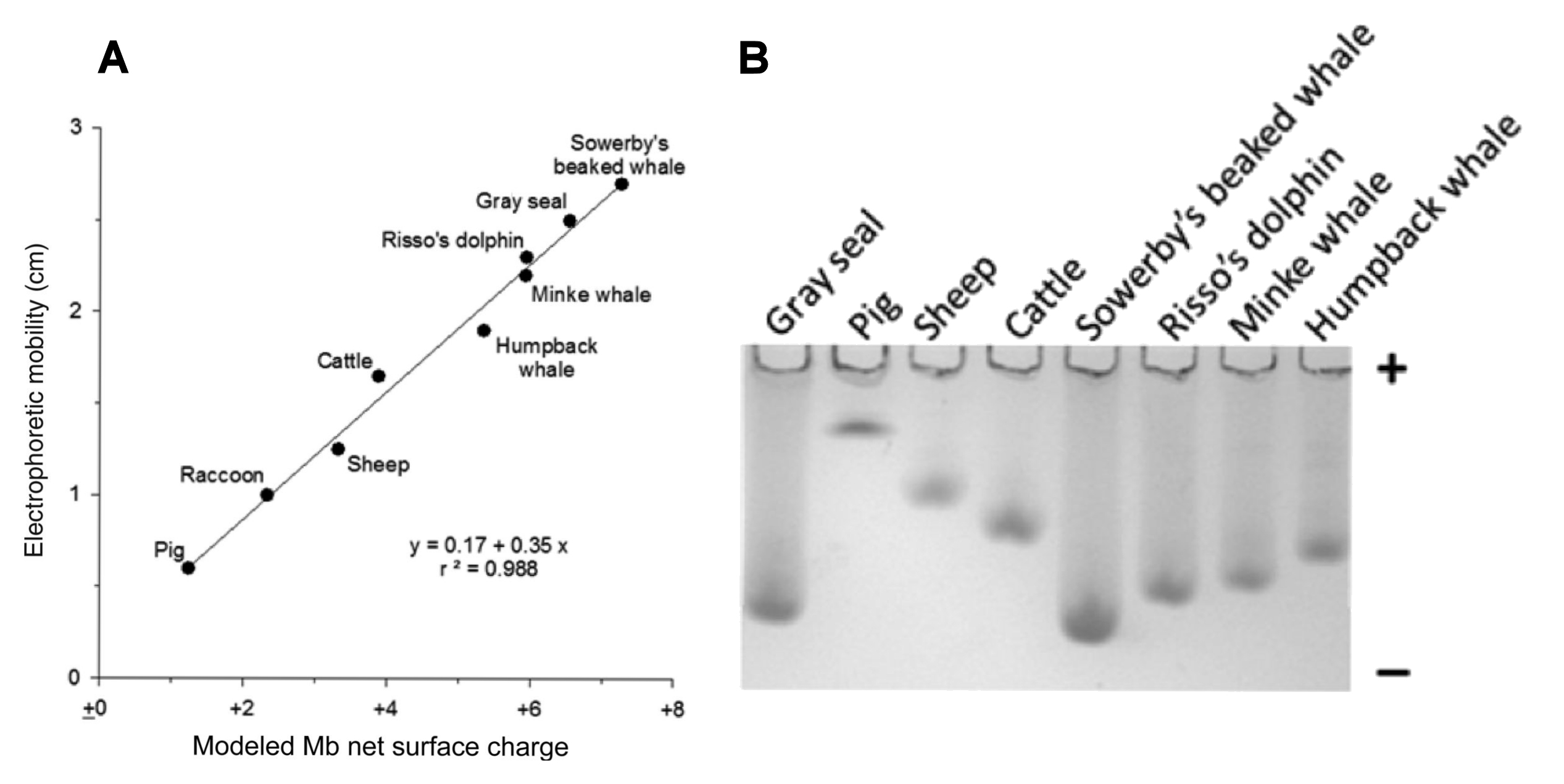
Protein electrophoretic studies [right] show that myoglobins in marine mammals (seals, whales, dolphins) have greater positive net surface charges than terrestrial mammals, as seen in their faster anodal mobility [towards the negative pole] relative to terrestrial mammals. This has evolved in parallel in seals (left) [Pinnipedia] and in toothed and baleen whales (right) [Odontoceti & Mysticeti]. Physiologically this is consistent with a higher O2 retention.
Protein electrophoretic studies [right] show that myoglobins in marine mammals (seals, whales, dolphins) have greater positive net surface charges than terrestrial mammals, as seen in their faster anodal mobility [towards the negative pole] relative to terrestrial mammals. This has evolved in parallel in seals (left) [Pinnipedia] and in toothed and baleen whales (right) [Odontoceti & Mysticeti]. Physiologically this is consistent with a higher O2 retention.