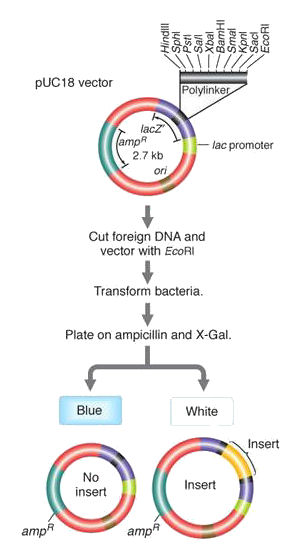

Selection scheme for recombinant plasmids
The plasmid vector
includes (1) an ampR gene for
resistance to the antibiotic ampicillin,
(2) a polylinker region
that contains a number of unique restriction endonuclease
recognition sites, located inside a (3) LacZ gene
that allows the plasmid to metabolize the sugar X-galactose (X-Gal) and produce a blue
by-product.
A foreign DNA is cut with one of the endonucleases in the polylinker, and recombined with a linearized plasmid cut with the same enzyme. The plasmids are allowed to transform a population of bacteria. Of the very large number of bacteria in the experiment (106~9s), only a small fraction take up a plasmid, and of these only a few of those plasmid contain recombinant DNA. Rather than screen millions of bacteria individually, a two-stage selection scheme is employed initially to screen out untransformed bacteria, and then those without recombinant plasmids.
The bacteria are grown on a petri dish with ampicillin and X-galactose. (1) Only those cells that have an ampR gene from the plasmid can grow at all. (2) Of those that took up a plasmid, those that do not contain recombinant DNA have an intact polylinker in the lacZ gene, and are thus able to metabolize X-Gal, Alternatively, successful insertion of recombinant DNA into the polylinker disrupts the lacZ gene, such that it is non-functional. Blue and White colonies thus signal unsuccessful and successful creation of recombinant DNA, respectively.
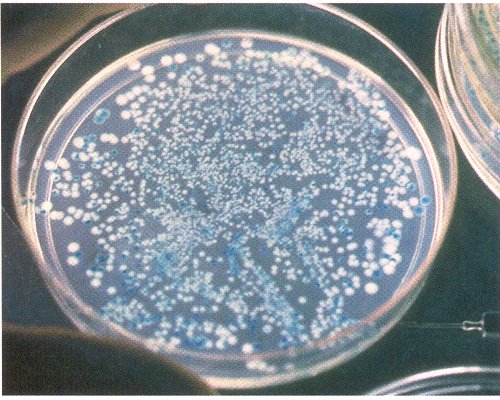
On the petri plate at right, multiple millions of bacteria do not grow at all (and are not seen), a few hundred blue colonies grow without recombinant inserts, and only a few dozen white colonies grow from a single cell that successfully took up the foreign DNA. Subsequent analysis will focus on these white clones.
A foreign DNA is cut with one of the endonucleases in the polylinker, and recombined with a linearized plasmid cut with the same enzyme. The plasmids are allowed to transform a population of bacteria. Of the very large number of bacteria in the experiment (106~9s), only a small fraction take up a plasmid, and of these only a few of those plasmid contain recombinant DNA. Rather than screen millions of bacteria individually, a two-stage selection scheme is employed initially to screen out untransformed bacteria, and then those without recombinant plasmids.
The bacteria are grown on a petri dish with ampicillin and X-galactose. (1) Only those cells that have an ampR gene from the plasmid can grow at all. (2) Of those that took up a plasmid, those that do not contain recombinant DNA have an intact polylinker in the lacZ gene, and are thus able to metabolize X-Gal, Alternatively, successful insertion of recombinant DNA into the polylinker disrupts the lacZ gene, such that it is non-functional. Blue and White colonies thus signal unsuccessful and successful creation of recombinant DNA, respectively.
On the petri plate at right, multiple millions of bacteria do not grow at all (and are not seen), a few hundred blue colonies grow without recombinant inserts, and only a few dozen white colonies grow from a single cell that successfully took up the foreign DNA. Subsequent analysis will focus on these white clones.