Use of Protein
Electrophoresis to detect Allozyme
variation:
Hemoglobin A versus
S


Use of Protein
Electrophoresis to detect Allozyme
variation:
Hemoglobin A versus
S
Substitution
mutations that result in
the
replacement of one amino acid by another with a different
electrical
charge
can lead to slight changes in the overall charges of the
protein. These
allelic variants (in DNA) give rise to protein variants called allozymes that
differ
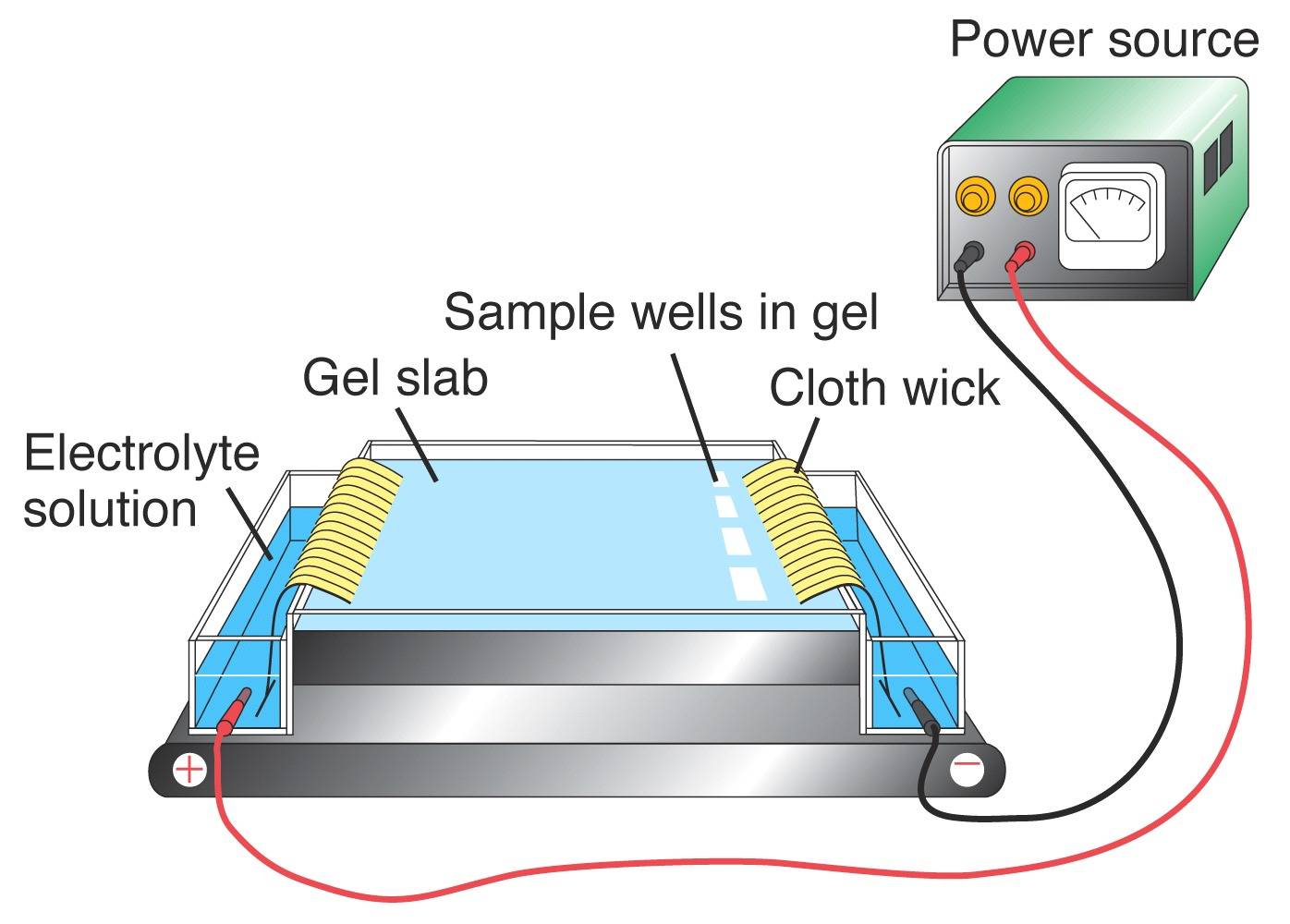
slightly in electrical charge. Protein
electrophoresis [left] is used to detect allozyme
variation. Tissue
extracts are introduced
into a
solid support medium (a gel)
in the sample wells at
the
Origin on the right-hand side of the gel. An electrical field is
applied: many proteins have a net negative charge and will
migrate from
the Origin at the cathode ("black"
=
"negative") end towards
the anode ("red" = "positive") end of the
field. The
positions of the protein products are detected either directly
by
staining, or by
coupled enzymatic reactions.
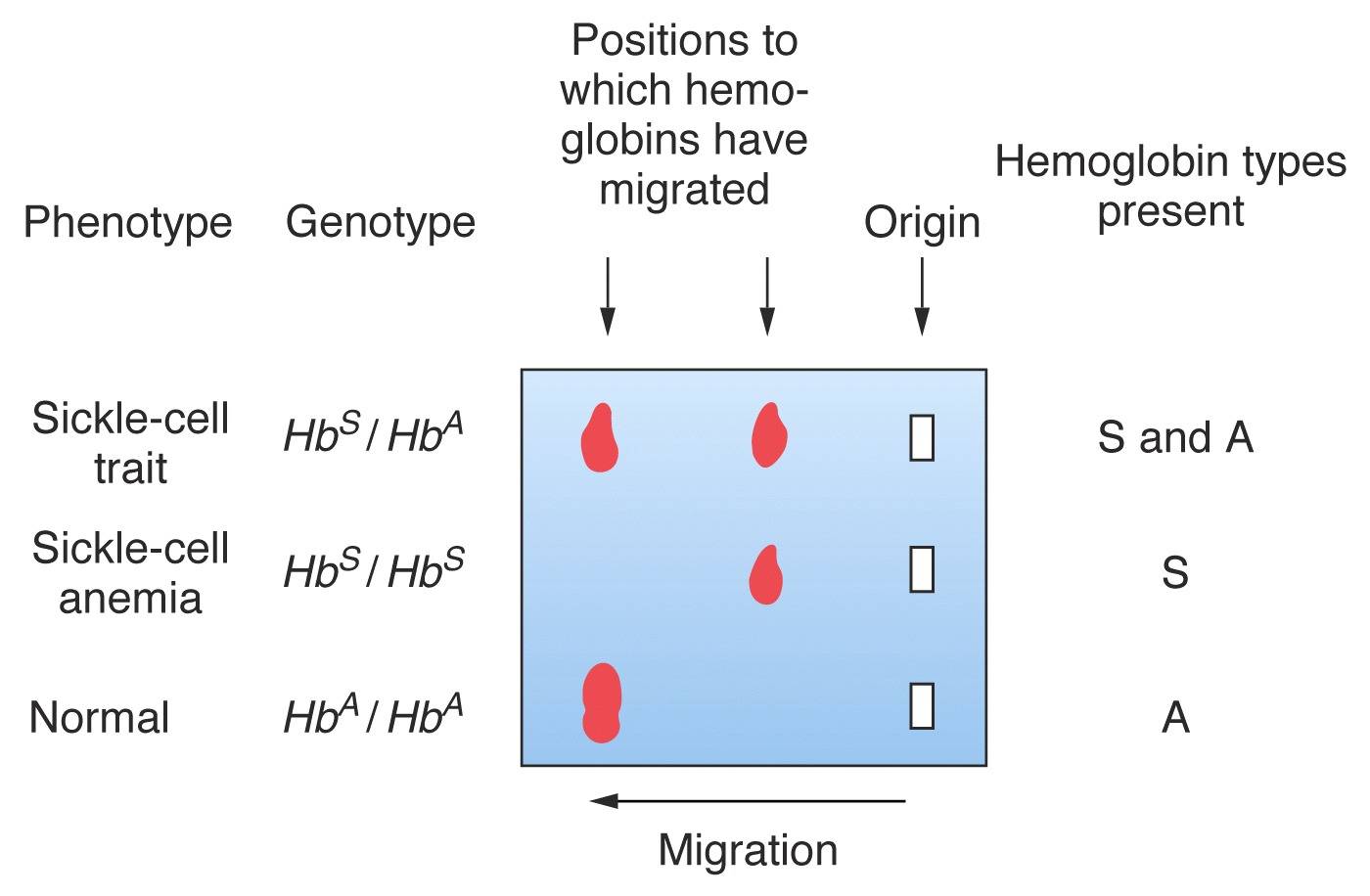
In the case of Sickle
Cell hemoglobin [right], replacement of a
negatively-charged Glu in
the standard HbA
beta-globin by a neutral Val in
HbS results in a
protein with a
slightly reduced negative charge. In homozygous
individuals, the HbA tetramer
electrophoreses
as a single "fast" band, and
the HbS tetramer as a
single "slow"
band.
Hemoglobin from a heterozygous
individual
(with both alleles)
comprises both forms
of the
tetramer, and therefore runs as two bands.
The gel would therefore be "scored" (from top to
bottom)
as FS, SS, and FF, indicating the presence
of S & A, S,
and A hemoglobin,
respectively. [Note that an SS
individual
has sickle-cell
anemia,
whereas an AS heterozygote
is
said to show sickle-cell trait].
Homework:
Critique the following
statement: "Electrophoresis
of Hemoglobin shows two alleles, F
and S,
for the Hb gene."