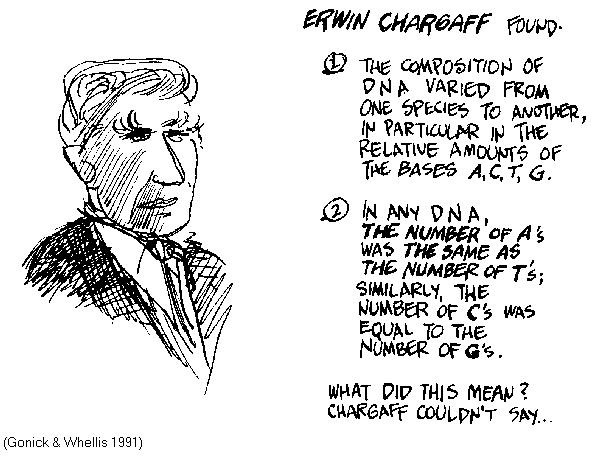
Chargaff's Rules

Given the accepted tetranucleotide hypothesis, most workers assumed that deviations from equimolar base ratios were due to experimental error. Erwin Chargaff (1905 - 2002) showed experimentally that base compositions actually varied among species. Within any one species, however, the molar ratios [A] = [T] and [C] = [G] are constant, and the proportion of [C+G] < [ A+T ]. These ratios have since been referred to as "Chargaff's Rules".
The molar
equivalences of A vs
T and C vs
G intuitively suggest some sort of pairing relationship.
Chargaff himself refused to speculate on the implications of his
empirical observations in the absence of further experimental
evidence, and regarded Watson & Crick's model building as a
scientifically unsound approach. Chargaff subsequently
denigrated molecular biology generally, and became embittered
over what he regarded as failure to acknowledge the importance
of his data. In interviews, Chargaff somewhat exasperatedly says
in effect, Yes I discovered the pairing of AT and CG,
No I did not discover base pairing.
HOMEWORK: Several generations of genetics students have said to themselves, "Two strands or three, bases inside or outside, and you have Chargaff's Rules - how long can it take?" Is this an accurate statement of the state of things at Cambridge in 1952?