Stereoisometry
of
Amino
Acids

Stereoisometry
of
Amino
Acids
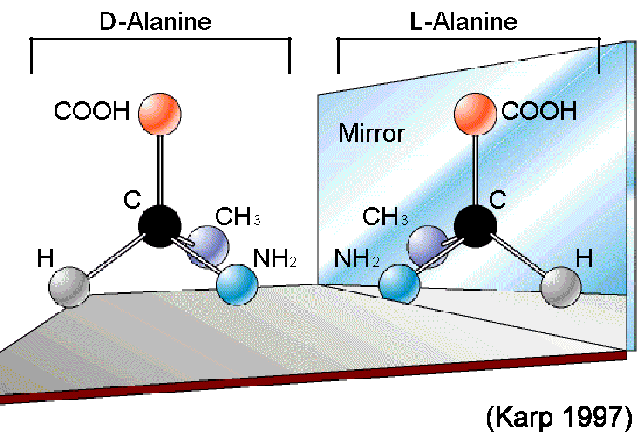
The four bonds of
the central (alpha) carbon
(C) of an
amino acid are directed towards the four corners of a tetrahedron. With
respect to the carboxyl (COOH) and amino
(NH2) groups,
there
are two possible arrangements of the H and Radical
group.
These arrangement are literally mirror images of each other, and
are
called
stereoisomers (enantiomers). Stereoisomers
are
designated D
(dextro-rotatory)
or
L
(levo-rotatory)
according
to the direction in which the crystalline forms rotate
polarized light, to the right and left, respectively.
Naturally-occurring proteins
comprise exclusively the L
forms of
amino acids.
In this
example, Alanine has a
CH3 as a radical group. If you
imagine
holding the model with the COOH at the
top and the NH2
at the bottom, the CH3 radical group in the D
form
will
be on your right. In
the
mirror image L form, it will
be on the left.
For an interesting science fiction treatments
of possible consequences of stereoisometry, see the 1969 film "Journey to
the Far
Side of the Sun."