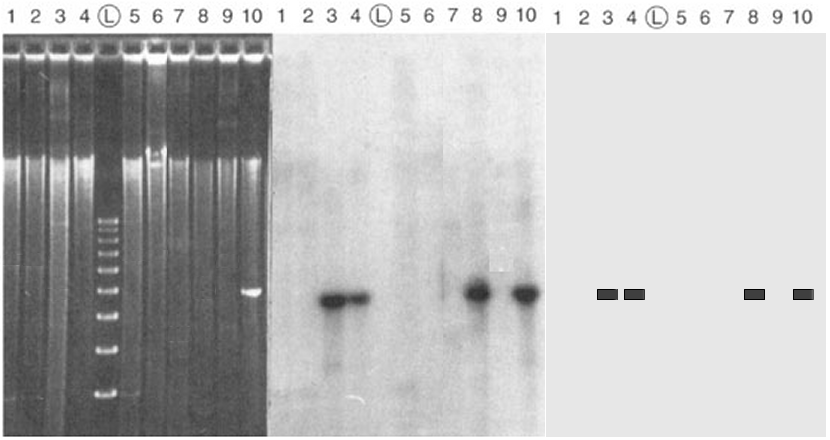
Southern Blot analysis of DNA

Southern Blot analysis of DNA
The left panel is an electrophoretic gel stained with ethidium bromide. Total DNA has been extracted from nine bacterial DNA clones (lanes ##1-9), digested with a particular restriction endonuclease, and separated by electrophoresis. Starting about one-third of the way from the top, a bright smear is present in each lane, indicating the presence of a random collection of restriction fragments of various sizes. There are no discrete bands, as no one DNA sequence is present in more than one copy in a haploid bacterium. The lane marked (L) is a molecular weight standard (a "Ladder"), with a series of DNA fragments at 100 bp intervals. A cloned DNA fragment has been loaded in lane #10: its mobility corresponding to the four rung of the ladder indicates a size of about 400 bp.
The middle panel
is a Southern Blot autoradiogram of
the same gel. The DNA in the gel was transferred
("blotted") to a filter. The filter was then hybridized
with a radioactively-labelled DNA (a "probe")
made
from the DNA loaded in
lane #10. The filter is then overlaid with a piece of X-ray
film. Where the probe DNA finds a complementary
sequence in the blot, it base-pairs ("sticks") to that DNA.
The radioactivity exposes the film at that point, and produces a
dark band.
The right
panel is a schematic representation of the autoradiogram. The
information content in this experiment is the presence or
absence of bands, and
the size
of the fragments, in each
lane. The bands in the autoradiogram show that a DNA sequence homologous to the probe DNA is present in clones ## 3, 4, & 8,
with the expected size as indicated in lane #10.
The DNA is absent in clones ##1, 2, 5, & 9.
The probe sticks to itself in lane #10, as expected.
This analysis indicates that the gene of interest has been
successfully cloned in the first set of plasmids, which can now
be analyzed further.
Figure modified from © 2000 by Griffiths et al.; text material © 2024 by Steven M. Carr